By Miguel Rodas, Environmental Compliance Inspector, City of Los Angeles Sanitation & Environment

(PRNewsfoto/LA Sanitation)
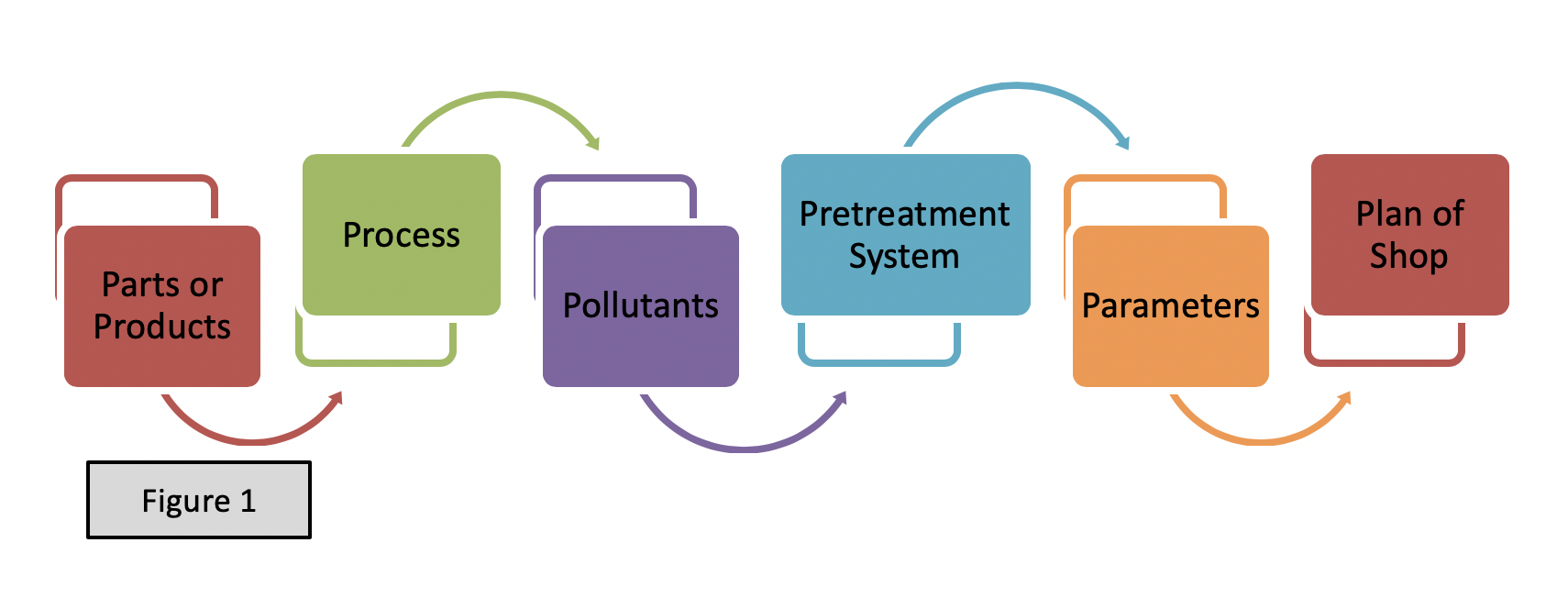
The article “Understanding Compliance Inspection Using the 6Ps Model” in the July 2018 issue of “Clean Water” magazine published by California Water Environment Association (CWEA) we stated that the 6Ps model serves as a navigational tool not only for inspectors, but also for industrial users (IUs). During a walkthrough-inspection of any facility, the 6Ps model serves as a guide to track any changes that needs to be addressed either by an IU or an inspector. We wrote that these Ps are not mutually exclusive, on the contrary one P leads to another P. This article focuses on the Pollutant(s) found in the metal finishing wastestreams. Because of that it is worth repeating the 6Ps here to keep you in perspective: Products, Processes, Pollutants, Pretreatment system, Parameters, and Plan of the shop. See Figure 1 below.
Let us first introduce a brief background on the metal finishing industry. The metal finishing industry sector is grouped under the North America Industry Code System (NAICS) with the 332813 code and it comprises with facilities engaged in electroplating, electroless plating (SIC code 3471), and anodizing, coating and coloring of metal parts (SIC 3479). The manufacturing of printed Circuits Boards (PCBs) is also include as metal finishing process (SIC 3672).
The metal finishing industry produces various undesirable wastes, solid and liquid because of the use of various type and volumes of chemicals known to be toxic in nature, which are utilized in the operations. The generation of waste-mainly in the form of rinses- is an important aspect addressed by pollution prevention initiatives for this industry. Understanding the pollutants in the wastestreams is extremely important since there are regulatory requirements to be fulfilled.
The enquiry by some IUs have revealed that there are two main characteristics governing and negatively affecting -at least compliance wise- the running of their businesses : (a) many of them started their business after being employees at another company and simply learned the dynamics of the metal finishing operations of the processes involved in the metal finishing operations without a clear understanding of the chemistry behind these processes; (ii) the core of the business (since the start of it) was exclusively profit-driven, without much knowledge of the regulatory challenges they could face while operating. In other words, the business started without a solid understanding of the chemistry behind each process and without a core integration of any environmental principles. These two overlooked aspects of business operations has paved a bumpy road that have brought some of these facilities to classified as being in “Significant Non-Compliance” (SNC[1]) at one point and consequently facing costly financial burdens associated with the enforcement orders. Some facilities have even closed after having facing enforcement and criminal actions by the local Publicly Owned Treatment Works (POTW). In order to help IU with their overall regulatory responsibilities, this article will focus on the identification of the pollutants in the wastestream, their sources and characteristics.
[1] The definition of Significant non-compliance (SNC) is found at [40 CFR 403.8(f) (2) (viii)]. SNC criteria are statistically-based, thus, the sooner the industrial user knows about the violation, and takes corrective action, it may have the opportunity to collect more samples in the reporting period, increasing their chances of avoiding being in SNC.
Before we take a good look at the pollutant, let us consider a popular phrase that is usually thrown out there: “We look but we don’t see” or even further “we see but we don’t observe.” The subject of observation for inspectors and IUs is indeed important because many times we often fail to see what is there be to be seen. Even when we observe what’s there, we fail to see what lies underneath. Now with that in mind let us proceed.
The Clean Water Act (CWA) states that “it is the national goal that the discharge of pollutants into the navigable waters (of US) be eliminated…”[1]. The objective of the CWA is “to restore and maintain the chemical, physical, and biological integrity of the nation’s waters”[2]. The CWA continues to explain that “in order to achieve” the above stated objective it was necessary to enact legislation aiming at the elimination of the pollutant from the navigable waters of the nation. Under the CWA, then, point source dischargers, direct and indirect dischargers[3] are required to obtain a permit to discharge and to comply with effluent limitations.
[1] https://www.law.cornell.edu/uscode/text/33/1251
[2] Ibid.
[3] Direct discharges are those facilities allowed to discharge directly to waters of the US. Indirect dischargers are those discharging through a Publicly Owned Treatment Works (POTWs).
Wastewater Characteristics
Now with our lens focused on the pollutant, let us take a look at the chemicals used in this industry. The metal finishing industry is notoriously known for the undesirable waste that produces, due first to the volume and secondly, due to the type of chemicals used in the operations. Some of the chemicals, to name a few, include nitric acid, sulfuric acid, fluoboric acid, hydrochloric acid, chromic acid, phosphoric acid, cyanide, and other alkaline chemicals which are corrosive enough to produce waste that meet the criteria of being hazardous waste and a threat to public health and the environment. These chemicals, indeed, have the capacity to impact the three main environmental systems: air, water and land.
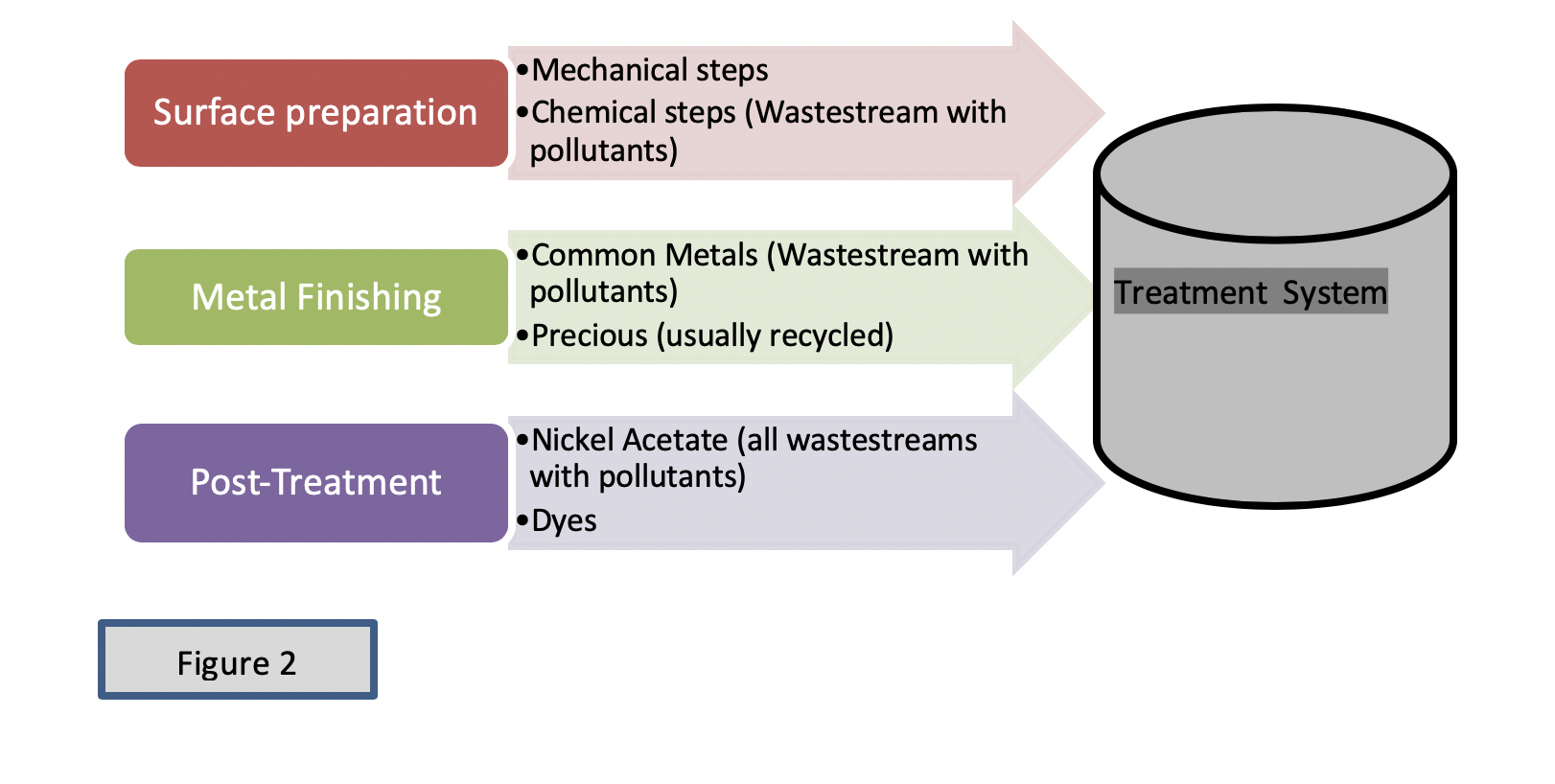
Wastes- in the form of rinses- originate at three steps involved in the finishing of parts (Bennett 1996): (a) surface preparation, (b) the finishing, and (c) the post-treatment of parts. The rinsing of parts is the primary source of industrial wastewater, and it contains the pollutants. Determining the pollutants present in the wastestream is one of the primary tasks of any enforcer or IU. Initial finding of pollutants can be done by first reviewing the permit application (in the case of new permittees) and all other documents submitted by an IU. Field inspections can reveal additional information wastestreams if an IU is an already existing permittee. During field inspections opportunities will arise to first have a qualitative assessment of the wastestreams (cloudiness, turbidity, color, etc.). Taking a close look of the pollutants involve several skills and close observations of processes and operations at a facility.
Surface Preparation
Although there are variations in the way facilities perform their finishing of parts, the processing of parts is generally the same (Bennett 1996): the part is cleaned (surface preparation), plated (finished), and the post-treatment of parts. Preparation of the surface of metal parts for subsequent steps is achieved using mechanical and chemical techniques. Pollutants are generated during the surface preparation step using mechanical techniques. This involves the mechanical removal of defects from parts, and they may include machining, grinding, sand blasting, filing, polishing, and buffing. The waste generated (pollutants) in the mechanical preparation of parts contains a mixture of buffing lint, other materials and metals (from substrate of parts). This waste is dry; however, it has to be lawfully manifested for off-site disposal.
Some of the substrate metals may remain and may pass onto the next step of surface preparation or the chemical cleaning. This step utilizes chemicals such as organic solvents, strong acids and high alkaline detergents. The pollutants encountered in the chemical surface preparations of parts include oil & grease (O & G), oxides and scales (from rusted parts), surfactants (TTOs), VOCs (acetone), low/high pH from acidic/alkaline cleaners, polishing/buffing residues (from the mechanical surface preparation step) and dirt removed off of parts. Once the parts are technically cleaned -mechanically and chemically, they are ready for any of the next finishing steps. The rinsing process during the chemical preparations of parts constitutes one of the primary sources of pollutants that will need treatment.
The Finishing Process
Now the parts are ready for the next step, the finishing. The six primary finishing processes that qualify or meet the criteria to be subject to the metal finishing effluent limitations include electroplating, electroless plating, anodizing, coating (phosphating, chromating, coloring), chemical milling and etching and manufacturing of printed circuit boards (EPA 1984). All the six primary processes require the three steps mentioned above (Surface preparation, finishing, and post treatment) in order to produce a good finishing. The manufacturing of printed circuit boards produces various types of pollutants that can be different than the other primary processes. If a facility performs one of these six primary processes, they will generate wastestreams deemed regulated wastestreams and therefore subject to the Effluent Limitation Guidelines (ELGs) under the Federal Metal Finishing category known as 40 CFR 413 and 433 (US 2016).
Pollutants generated during the actual finishing or plating of parts (any of the six-primary processes). Rinsing removes the chemicals (metals and other bath additives) contained in the plating solutions used in each process. Rinses may contain low or high concentration of the chemicals (depending on how well dragouts is implemented), which are components of the process tanks. Eventually, some of the process tanks may get contaminated if not maintained properly. These are concentrated sources of pollutants, which need to be handled differently and appropriately when needed.
The pollutants encountered in the rinses of the electroplating or electroless plating of parts depending on the type of process tanks include cadmium, copper, nickel, chromium, zinc (common metals), or silver, gold and palladium to mention some of precious metals. In addition, some process solutions may contain cyanide. The pollutants found in the rinses of processes such as anodizing may be of two types: chromium-laden rinses, if the process involves using chromic acid or acidic if they contain sulfuric acid. The pollutants found in the coating may be chromium (from chromate salts) such as sodium or potassium dichromate, or acidic solutions (low pH) from phosphoric acid contained in proprietary chemicals or toxic organics from passivation, and coloring dyes and pigments used in the coloring of parts. Pollutants found in processes such as chemical milling & etching are metals removed from parts in order to produce the desired design in parts. Depending on the substrate of parts (stainless steel, brass, and aluminum), metals found in rinses may be chromium, cadmium, nickel, copper, etc. This type of operation also includes the stripping of metallic coatings. The manufacturing of printed circuit boards uses copper solutions and tin/lead solutions. These metals may be found in the rinses used in this particular sector. Of particular interest are the cyanide-laden rinses used in certain type of metal plating solution, such as zinc, cadmium, silver or gold. In general, numerous pollutants such as salts containing metals, cyanide, chrome-laden rinses, acidic/bases, and dissolved basis materials may enter into the rinses that will constitute the wastewater to be treated.
Post-treatment of Parts
Some of the post-treatment processes include nickel acetate sealing or sealing solution that contains hexavalent chromic acid. Although in low concentration, nickel may be found in the post-treatment rinses. Some facilities may be engaged in applying a final chromated dye into parts, some of which may contain silver such in black chromate.
Depending on the sources of wastestream handled at the facility, you may have a very complex chemical mixture that can be difficult to treat if not handle appropriately. Although this paper is not about wastewater treatment, the way EPA prescribes treatment is, first, by proper segregation of all sources, then removal of oil & grease and neutralization of the wastestream generated in the chemical cleaning step. Secondly, reduction of chrome-laden wastestreams (if any), oxidation of cyanide laden rinses (if any), and finally hydroxide metal precipitation when all the above wastestreams have been properly segregated (US 2016, 2-10). See figure 2 of all pollutant sources.
Final words
We stated at the beginning of this article that many IUs start their businesses by simply following (empirically) the dynamics of the processes involved in the metal finishing operations without a clear understanding of the chemistry behind. Thus, because their base-knowledge is limited, this limitation has its implications. Even when many IUs may have some knowledge (often insufficient) regarding wastewater treatment it lacks the intrinsic technicality associated with chemistry. First, IUs only know what others are doing (empirically) and so they follow what others do. Second, the major hindrance is not-knowing what pollutants are present in the wastestream, and their proper treatment.
Finding the pollutants can be exacerbated by other factors as well. As new chemical process formulations are most often proprietary, chemical suppliers guard their specialized formulations as trade secrets. Very often this proprietary formulation contains TTOs that may appear in the final effluent, untreated, thus violating the effluent limitations. Relying entirely on information supplied by chemical vendors has also its implications: IUs do not know what the constituents in the chemicals they buy are. Many of the most common discharge violations besides heavy metals, and cyanide, are TTOs violations that may come from the proprietary components of chemicals additives added to process tanks.
We also stated that the lack of any environmental management system into the core of the business contributes greatly into the ultimate demise of a facility. This deficiency is usually observed in poor housekeeping practices (wet floor, spills, etc.), which is another implication that metal finishers face and thus contributes to the overall generation of hazardous waste. This hazardous waste requires proper treatment and disposal. This situation compounds the difficulty of meeting compliance with the effluent limitations.
To conclude, both POTWs and IUs have requirements and responsibilities[1]. However, the Compliance business does not have to be a grim duty, but an exciting endeavor for both. For this reason, this article has the educational objective to reach out to all metal finishers, especially those within the jurisdiction of City of Los Angeles. The sustainability of metal finishers is one the primary goal of the LA Industry Program, which is an ongoing effort to explore strategies and opportunities to cultivate a business-friendly environment for businesses.
[1] 40 CFR 403.5 applies to IUs; 403.8 apply to POTWs.
Miguel Rodas, author of this article acknowledges the contribution of Environmental Compliance inspectors (ECIs) Minh Guyen, and Jan Marte for grammar check and editing of this article; Manik Mohandas Engineering Associate (EEA) for checking of content and editing as well. Special thanks to Masresha Girmachew ([email protected]) for her professional grammar check & editing as well.
Bennett, Pat. Assessment of The Metal Finishing and Plating Industry Source Reduction Planning Efforts. DTSC, 1996.
EPA, U.S. “Guidance Manual for Electroplating and Metal Finishing Pretreatment Standards.” Washington, DC: Office of Water, february 1984.
US, EPA. Preliminary Study of the Metal Finishing Category 2015 Status Report. Preliminary Study, US EPA, Washington DC: US EPA, 2016.