View CWEA’s PFAS resources page, support by Carollo.
It is a fact that chemistry has changed our world for the better. It has played an important role in the production of commercial products, thus making a great variety of raw materials accessible to manufacturers across all industrial sectors and leading to the production of many consumer products. However, we hear more and more about the other side of the coin: chemicals of emergent concern affecting human health and the environment. We are referring to the Perfluoroalkyl and Polyfluoroalkyl Substances, shortened to PFAS. The EPA1 estimates that there are thousands of these compounds, so they are referred collectively as the PFAS family.
What makes PFAS the subject of our attention? PFAS are components of a great variety of consumer products, and they are negatively impacting all of us and the environment. Consequently, understanding what PFAS are is of paramount importance. Not only are they known as chemicals of concern because of their impact on water, land, ecosystems, and humans, but they are now also known as the ‘forever chemicals’ because of their high resistance to breaking down in the environment and thus becoming malignly ubiquitous across the environment.
The PFAS Family
PFAS is a large family of chemicals used in the manufacturing of various consumer products. Well known sources indicate various figures as to how many PFAS have been synthetized and how many are in various products. As recently as 2022, the estimated figure is at more than 9,000 compounds. The reality is that they now form part of our conveniences, and we find them in the foams used in fire-fighting, electronics resistant to water, grease-proof food wrappers, clothes resistant to rain, carpets resistant to stains; cosmetics, and house cleaners (Levy, 2020). Chemically speaking, although each PFAS molecule is different, the main chemical backbone of their molecule is composed of carbon and fluorine atoms, synthetically forced to a chemical bond that is mostly utilitarian, or at least profitable. Despite the possible good uses of PFAS, they do have a downside: they have been manufactured for use in so many consumer products that they have been detected in the three major environmental compartments: water, air, and land…including humans.
The Nature of Bonding
Human bonding and chemical bonding shares some similarities. In the human realm, bonding, within a family for example, is based on shared feelings, experiences, love, and even cultural heritages. The interactions between humans can be positive or negative, creating a polarization that may produce tension. Initially, we may may enjoy an apparent relational stability. However, the bonding loses its stability as polarization increases due to conflicts or incompatibilities. In short, human bonding does not last long…definitively they are not forever.
In the chemical realm, a chemical bond holds together atoms but by other means: attractive forces based on electronegativity, forcibly engineered for economic reasons. Chemical bonding is based on the sharing of electrons between two or more elements, as each element involved achieves their stability by either losing or gaining electrons. Chemical elements accomplish their utmost stability by completing their last electronic shell with electrons. In a PFAS molecule, there are a number of bonds between carbon (C) and fluorine (F); Figure 1 shows the C-F bond and the polarization between them, while Figure 2 shows a PFAS molecule.

Figure 1
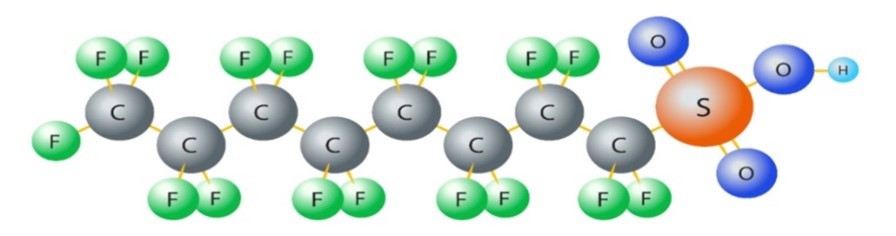
Figure 2
Fluorine, however, exerts a stronger tendency to possess all the electrons. Like humans who tend to keep all feelings or material possessions (selfishly) close to heart, fluorine keeps the shared electrons close to its nucleus. The shorter, stronger, and more stable molecule created is almost impossible to break. While poor bonding in the human realm translate to bad relationships and strong, stable bonding translate to good family relationships, it is worth making a contrast in that the strong, stable bond between carbon and fluorine in PFAS molecules is not a good relationship, as they ultimately lead to both the “forever” nature of these chemicals, and their negative impacts on the environment.
Nature knows and recognizes its own kind
Nature knows and recognizes its own kind of molecules; the synthetically-designed bonds between carbon and fluorine renders the entire molecule of PFAS of being commercially attractive. Yes, PFAS compounds have extensive attractive (if not necessarily good) applications in industry and have become components or ingredients of products used in our daily life. Unfortunately, as Ruan et. al, 2022 state, “the enduring environmental legacy of PFAS will remain long after they are discontinued.” The question is: at what cost?
Human Health and the Environmental Effects
The cost that PFAS has brought to us is the damage to the environment and to human health. This damage is reported by the CDC as PFAS chemicals are being detected in our bodies. According to the CDC, exposure to PFAS occurs by consuming PFAS-contaminated water or food. In fact, the EPA reports that PFAS are the subject of scrutiny due to their ubiquitous presence in soils, drinking water sources, different biological species, and humans. What are the effects? The CDC states that studies indicate that besides affecting thyroid function, PFAS affect reproduction, development, growth, and the immune system. Furthermore, the CDC reports finding four specific PFAS in blood samples of nearly all people tested. The EPA goes on to state, “it is because of their widespread use [that] many PFAS are found in the blood of people and animals all over the world.”
Bonds function to hold humans as well as chemical elements together. Human bonding is not forever; it is an established fate. Nature knows how to handle us; it breaks all of us down without any problem. The bonding in PFAS, on the other hand, were synthetically created to last, ignoring fundamental principles in nature. Can humans outsmart nature? No. Today, we are dealing not only with the hazardousness pervasiveness of PFAS (including other chemicals), but also with their undesirable, persistent, and ill foreverness. As stated by these authors Ruan et. al, 2022. the legacy of the forever chemicals is here to stay–literally forever. In the meantime, nature continues to groan with pains because of its inability to degrade them. Because of the hazardous, toxic, and ubiquitous presence of PFAS across all environmental media, we need to be informed of the impact to human health. Our position should, therefore, be for more coordination between manufacturers, government, and community stakeholders for a progressive phase-out of PFAS as ingredients in consumer products.
References
Levy, M. G. (2020, December). The Chemistry of Convenience. ChemMatters, pp. 5-7.
Ruan, T., Field, J., Cousins, I., Lohmann, R., & Jiang, G. (2022, May 17th). Emerging Contaminants: Flourinated Alternatives to Existing PFAS. Environmnetal Sciences & Technology, 56 (10).